News
A Micro Mind Reveals Macro Answers About the Human Brain
January 17, 2017
Why do humans do what they do? What is it that makes us happy or depressed? Understanding how the human brain functions is a complex matter. Just think, a human brain has 86 billion neurons with trillions of unmapped connections; identifying them all is a herculean task.
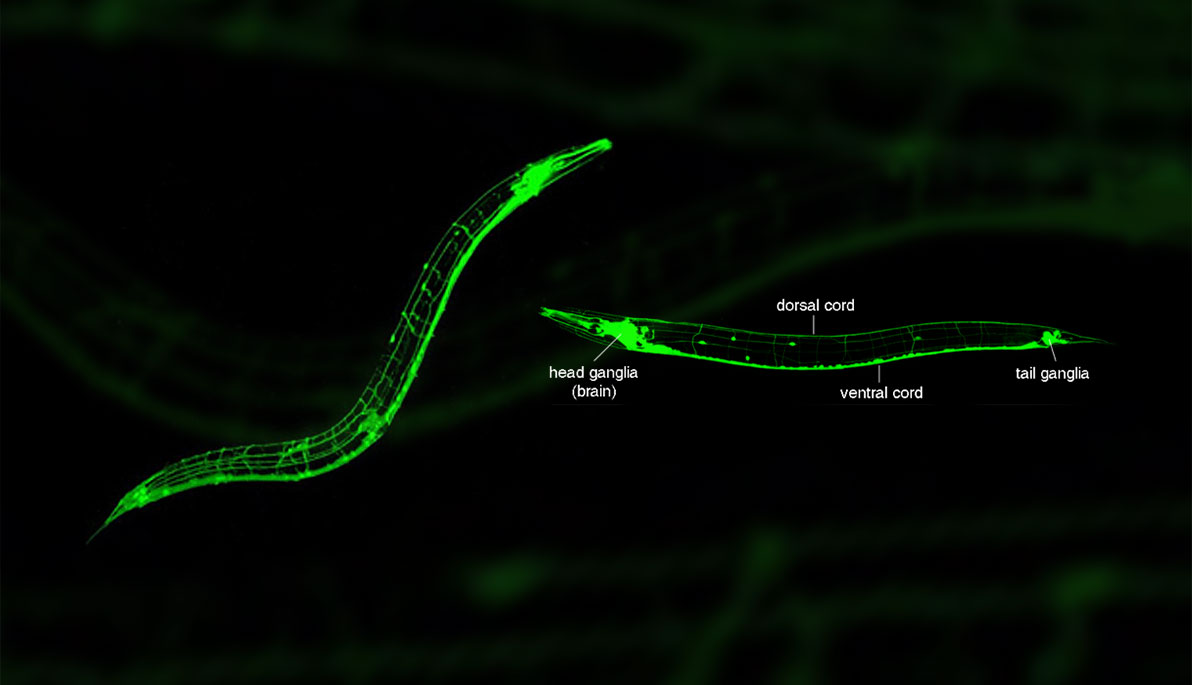
To gain a better grasp of how the human brain works, Navin Pokala, assistant professor of Life Sciences at the College of Arts and Sciences, teamed up with researchers at Caltech to study a different sort of mind: the brain of the microscopic nematode Caenorhabditis elegans (C. elegans), otherwise known as the roundworm.
A roundworm’s brain is much simpler than a human’s—with only 302 neurons and a completely mapped neural wiring of 6,000 connections. It is the closest biological analog to an electronic circuit board. Engineers design and study circuits using breadboards that allow circuit elements to be added, removed, or modified. This method can be implemented in biology to understand how neural circuits in the brain work and allow scientists to manipulate the activity of neurons.
“The nervous system of the roundworm C. elegans is the See Spot Run of neuroscience,” said Pokala, whose paper was published in Nature Methods in December. “Lessons learned from trying to comprehend its tiny brain will undoubtedly be useful for understanding the staggeringly more complex ‘Complete Works of William Shakespeare’ that is the human brain.”
Roundworms exhibit behaviors ranging from simple reflexes to searching for food when hungry to learning to avoid foods that previously made them ill, as well as social behavior. Pokala will use the ringworm’s biological breadboard as a way to develop computational algorithms and experimental strategies for understanding and predicting how neural circuits generate behaviors. “We would like to understand how the networks of neurons in the brain produce behaviors and emotions, and how to best intervene when those networks produce the undesired behaviors and emotions of mental illnesses,” he explained. Ultimately, these findings could be used to help patients suffering from psychiatric illnesses.
“We have prototyped a diverse set of genetically-encoded light and chemical-activated tools for manipulating and perturbing the activity of individual C. elegans neurons,” explained Pokala. To comprehensively measure behaviors as they change over time, Pokala and the team developed a software suite for tracking dozens of animals simultaneously for a spectrum of locomotion behaviors. They are using these tools to test algorithms for reverse-engineering the nervous system. “The gal4-UAS system described in our recent paper allows us to turn specific C. elegans neurons on and off, much like an electrode being used to probe elements of an electronic circuit board,” he said.
This database will be a powerful basis for developing and testing mathematical models of how the nervous system functions. “We hope that these algorithms can be applied to the human nervous system in order to develop more effective therapies for psychiatric illnesses.”